Modifying a Protocol
Modifications
***After Initial Approval: Remember to Submit ALL Requested Changes for IRB Approval!!!***
All studies approved by the UTA IRB require that modification requests must be submitted and approved in Mentis before any changes from the approved version of the protocol (and associated protocol documents) are implemented. This applies to studies determined to be exempt; studies approved through the MR or GMR “flex” internal review pathways; and studies approved at the Expedited or Full Board level per the federal regulations. Failure to obtain IRB approval for changes prior to implementation is considered both a protocol violation and noncompliance.
Possible modifications include, but are not limited to, procedural changes to a protocol; addition or removal of study sites or recruitment methods; changing the PI or Faculty Advisor; requesting additional participants beyond the approved number; reporting changes in funding; and changes in approved study documents or materials, including Informed Consent Document(s) and recruitment materials. **Changes to the PI, Faculty Advisor, Specially Qualified Personnel (SQP), and Non-UTA Personnel require IRB approval via a Modification. Other personnel changes are not required to be submitted as a Modification and instead are the responsibility of the Principal Investigator to track, verify training, and maintain documentation. See this page for more details.**
Step 1: Begin a Modification in Mentis
Go to the IRB home page and select “Modify an Existing Protocol Or Submit a Continuing Review for an Existing Protocol” then click “Proceed.”

Step 2: Select the Protocol you Wish to Modify
Select the protocol from those presented and complete all sections of the modification page and select the “View” button.

Step 3: Start a modification review
Click the button “Start Modification Review” to begin a modification.

Step 4: Complete the Mentis Modification Page in its Entirety
In section #3, check the box(es) indicating any issues or document(s) that you want to modify. After you click a box, a window will appear where you can enter in an explanation of each change/edit. You can check multiple—or even all the boxes—for a modification as needed. Click “Save” when you are finished completing this section.
It is essential to list and describe all modifications being requested in #3 including any proposed changes to approved documents, procedures, inclusion/exclusion criteria, research sites, personnel (PI/FA), etc. Only the changes specifically listed in section #3 will be reviewed by the IRB. If not enough information is listed in #3, the modification will be returned to you for edits.
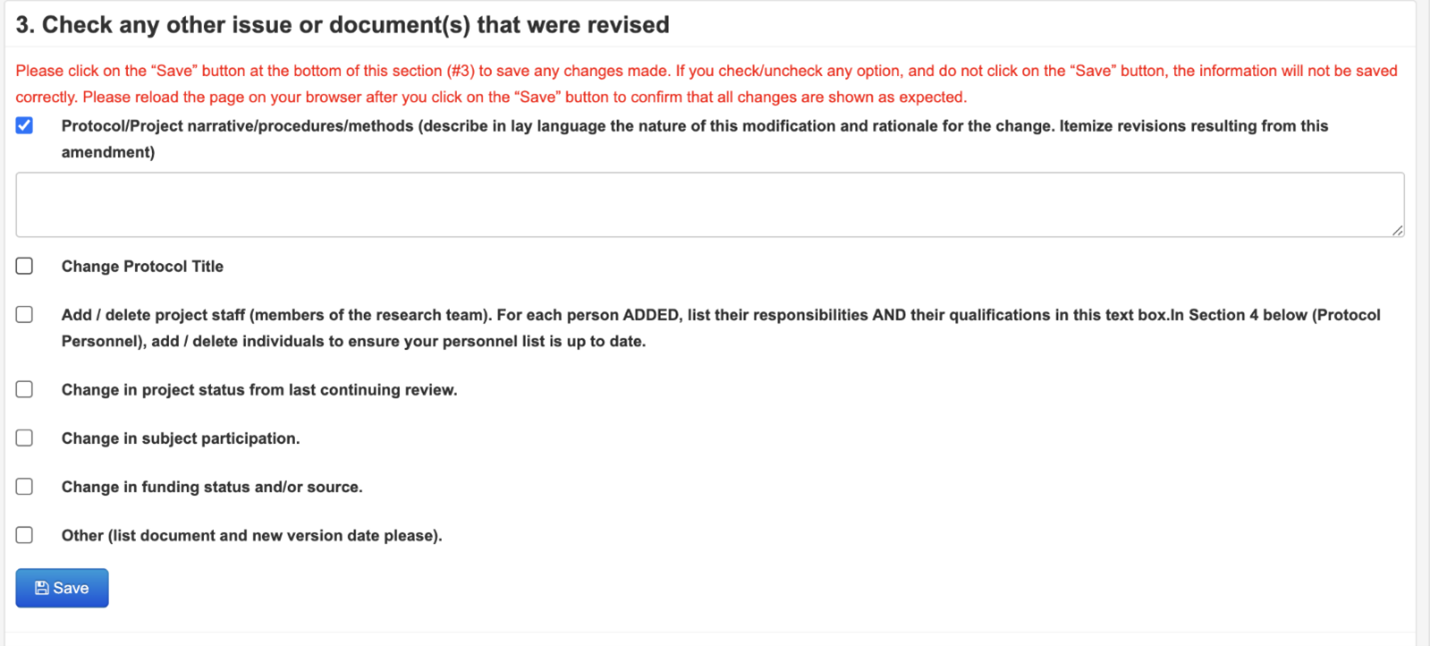
Step 5: (Optional, only if you are changing research personnel) Update the list of protocol personnel
**Please note that not all personnel changes require approval by the IRB via a Modification. See this page for more details.**
If you are requesting a change of the PI, Faculty Advisor, Specially Qualified Personnel (SQP), or Non-UTA Personnel, in item #4 click the “Add Personnel” or the “delete” button to modify your list of personnel. You may also delete personnel here by clicking the “delete” button. Please note that you must also address these changes in #3 and list who is being added and deleted by name.
Step 6: Update any Risks to Participants
Section #5 asks about changes to risks. If your modification does not change the risks presented to subjects you can select “N/A” from the dropdown menu. If the changes will involve a change in risks, explain how this information will be communicated to currently enrolled participants.
Please note, not all modifications involve a change in risk level. In fact, most modifications don’t change the risk level at all and may not need to be communicated to participants.

Step 7: Consider use of Forms/Templates
In Section #6 you will find a link to IRB consent templates and the protocol application form. You don’t need to upload anything to this section—just use the link if you need to find a blank IRB form or template!
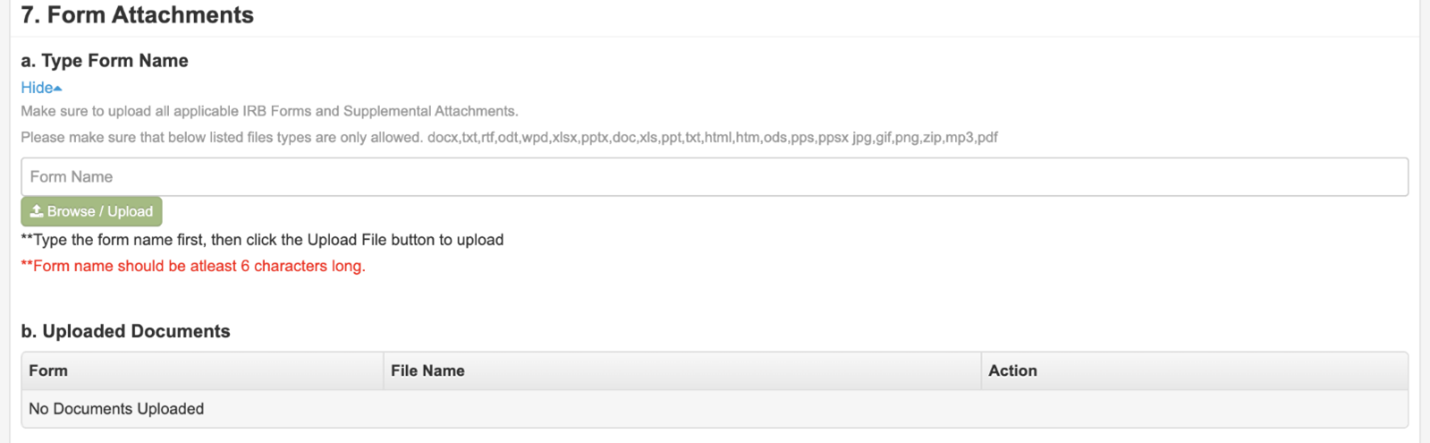
Step 8: Attach All Documents that have been Modified
In section #7 upload any relevant documentation such as the revised application form, consent form, or recruitment fliers. **When possible, use highlighting or tracked changes to indicate the proposed revisions.**
To upload all required forms and documents, type the name of the document in the white box and then click the green “Browse / Upload” button and select the desired form. Your files will not upload correctly if the file name is not typed in the white box first.
Note: your protocol application should always be updated to show how you are modifying your protocol.
Step 9: Save and Submit your Modification for IRB Review
1. Student Principal Investigator (UTA students): Click the button to “Submit to Faculty Advisor.” Your Faculty Advisor must review and approve your submission before it is sent to the IRB Coordinator. Once approved (please note they may send it back to you for revisions) they will click the button to “Submit to IRB Coordinator.” At this point, your protocol will be in the IRB Inbox/Queue for review. Note that the IRB Office does NOT see your study until your Faculty Advisor clicks the button to “Submit to IRB Coordinator.”
2. Principal Investigator/Faculty Advisor: The Faculty Advisor and the Principal Investigator (unless the PI is a student) can submit the modification directly to the IRB. Click “Submit to IRB Coordinator” to send the modification to the IRB.

3. Other Protocol Personnel: If you are protocol personnel other than the PI or Faculty Advisor, you will not be able to submit the modification directly to the IRB. It has to be routed to the study PI first. Click “Submit to Principal Investigator” to route the modification to the PI.

At this point your modification will be in the IRB Inbox/Queue for review.
Step 10: IRB Review Process
After your modification has been submitted, it will be reviewed under a specific Review Pathway according to funding/regulatory status and risk level. You can check on the status of your protocol in the Mentis IRB System under “Pending Protocols.”
Once the IRB Coordinator has conducted an initial review of your modification, if clarification or revisions are needed, it will be returned to you in the Mentis IRB System – you will receive an email from the “ERA Help Desk” which will let you know your modification has been returned. Please complete the revisions in their entirety ASAP and resubmit the revised documents within the Mentis IRB System.
Greater than Minimal Risk and Full Board modifications may be reviewed through expedited procedures or by going to the Full Board if the changes are major. Most other minor and administrative changes can be reviewed and approved by IRB Staff outside of Full Board meetings.
The process for review of Modifications is described in detail in the IRB’s Standard Operating Procedures, see section IX.B. The process is dependent upon the type of Modification as they are classified into three groups:
Major Modifications
Major modifications are changes that could increase the potential risks to study subjects or alter the risk/benefit assessment of the research. Major modifications may also involve a decrease in benefit or that otherwise result in alteration of the risk/benefit assessment of the research. Some examples of Major Modifications:
- Change of PI / FA (also requires a Change of PI form to complete, sign, and upload)” only when the incoming PI / FA does not possess the same or comparable expertise, qualifications, licensure, or experience as the current PI / FA
- Increasing the study population by more than 20% for Greater Than Minimal Risk studies
- Changes to the inclusion / exclusion criteria that may affect the risks and benefits
- Alterations in the dosage of an approved drug
- Adding a device to a study
Minor Modifications
Minor modifications are those which do not increase the probability or magnitude of risk to subjects. Some examples of Minor Modifications:
- Adding recruitment material or research tools that are not substantially similar to what has been previously approved by the IRB
- Increasing/decreasing the amount of compensation
- Revisions to the Informed Consent Document, recruitment materials, surveys or questionnaires
- Changes to the intervention or procedures
Administrative Modifications
Administrative modifications are a category of minor modifications that do not affect the study's risk level. Some examples of administrative modifications:
- Update to study title
- Update in study funding (grant congruency and impact on review pathway will be verified by IRB Staff)
- Deleting/adding items to a research tool, survey, or questionnaire, when the questions being added are substantially similar in nature to the previously approved version
- Adding recruitment material that is substantially similar to previously approved versions
- Administrative changes to consent form
Step 11: Official Approval
Once you receive notice from Mentis that your modification has been approved, you are good to go! You can find a copy of your IRB Approval Letter uploaded to your modification in Mentis.
If you have any questions during the modification process, please reach out to regulatoryservices@uta.edu and one of the IRB staff members will respond and assist you.
