Personnel Changes
IRB Update: Modifications No Longer Required for Most Personnel Changes
Modifications represent the highest volume of submissions received by the IRB, with a significant number of these requests pertaining to changes in personnel. Often, the personnel listed in these Modification submissions have not yet fulfilled their training requirements. As a result, the IRB staff must consistently follow up to notify them, check their status, and send reminders. The process is time-consuming and diverts time from reviewing other submissions in the IRB queue. Additionally, it is inconvenient for PIs to wait for IRB approval to add new personnel, especially when team members frequently change during the project.
To alleviate the administrative burden and enhance efficiency, the IRB has revised its requirements regarding personnel changes to approved protocols. Previously, all personnel changes necessitated IRB approval through a protocol modification. Effective immediately, IRB approval will only be required for certain personnel changes, including those involving the Principal Investigator (PI), Faculty Advisor, “Specially Qualified Personnel,” and Non-UTA personnel. Modifications related to other types of personnel will no longer require IRB submission or approval of a Modification. Instead, Principal Investigators (PIs) will be responsible for verifying training and conflict of interest (COI) requirements before personnel engage in the project, as well as for maintaining the necessary records. In addition to improving efficiency, this procedure is consistent with best practices at IRBs across the nation and ensures the IRB’s mission remains focused on the protection of human subjects participating in research. Please refer to the table in Section A below for an overview of PI responsibilities and instructions.
Section A. Summary Table – IRB Protocol Personnel Changes
| Personnel | Modification Approval Required? | PI Responsibilities | Procedures |
|---|---|---|---|
| PI | Yes | Obtain IRB approval prior to change | Submit a Modification request with Change Form for IRB approval |
| Faculty Advisor | Yes | Obtain IRB approval prior to change | Submit a Modification request with Change Form for IRB approval |
| Non-UTA Personnel | Yes | Obtain IRB approval prior to change and address requirements for a reliance agreement when applicable | Submit a Modification request for IRB approval and consider requirements for multi-site research / reliance agreement |
| Specially Qualified Personnel (UTA) | Maybe | Prior to the SQP engaging in the research, the PI is responsible for (1) performing a qualifications review for SQP, (2) verifying training/COI requirements are complete, and (3) maintaining appropriate documentation. Qualifications Review: The PI is responsible for ensuring that new personnel hold the same or comparable qualifications as the original approved personnel. |
Qualifications Review Review professional degrees, certifications, experience, and any other special qualifications necessary for the research to confirm they are sufficient and comparable to original personnel. If they match, a Modification is not required. If they do not match, submit a Modification request for IRB review. If unsure, contact Regulatory Services for assistance. Personnel Training Requirements*
|
| All Other UTA Personnel | No | Prior to the personnel engaging in the research, the PI is responsible for (1) verifying training/COI requirements are complete, and (2) maintaining appropriate documentation. | Personnel Training Requirements*
|
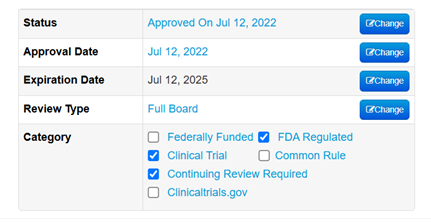
- To check if your protocol is Greater than Minimal Risk, Full Board, FDA Regulated, or a Clinical Trial, open the protocol in Mentis and view this box in the upper right corner of the page:
Section B. Impact of Personnel Changes on Approved Protocol Materials
PIs will need to consider whether a personnel change affects any other approved protocol-related materials such as the informed consent document or recruitment fliers. If these or any other materials need to be revised to reflect a personnel change, a Modification must be submitted for IRB approval. Typically, these types of modifications are administrative in nature and can be processed more quickly. Moving forward, it is recommended that protocol materials only include the contact information of the PI and/or Faculty Advisor so the materials will not need revision every time personnel changes are made.
Section C. Personnel Access to Protocols in Mentis
Since most new personnel will no longer be added to a protocol via Mentis, a new feature is currently under development where PIs will be able to share access permissions for their protocols and specify “view access” or “edit access” for each user. The “edit access” feature will allow the personnel to make submissions for the protocol. This new feature is expected to roll out in the summer. In the interim, please provide copies of the protocol or its relevant sections to your protocol personnel.
Section D. Monitoring and Non-Compliance
The IRB may request to review a protocol’s current personnel and their qualifications during routine Continuing Reviews or post-approval monitoring, or during investigations related to non-compliance or subject safety. Engaging personnel without proper vetting and documentation according to the requirements outlined above will be considered non-compliance with the IRB’s policies and procedures. Serious or continuing non-compliance is reported to a PI’s Department Chair and may result in additional restrictions imposed by the IRB, or suspension or termination of research approval.
Section E. FAQs
Who qualifies as protocol personnel?
Only individuals “engaged” in the research need to be protocol personnel. A person is engaged if they will: (1) interact with subjects (directly or indirectly), (2) intervene with subjects by performing procedures or manipulating their environment, (3) obtain informed consent from subjects, or (4) obtain/access/use identifiable information or biospecimens from any source.
A person is not engaged and does not need to be included as protocol personnel if their involvement is limited to: (1) providing commercial services typically performed for non-research purposes, without professional recognition or publication privileges, (2) providing prospective subjects with information about or availability of the research (e.g., copy of the consent, flier, or researchers’ contact information) but they do not obtain subjects’ consent, act as representatives of the investigators, or participate in the intervention, (3) releasing human subject information or biospecimens to UTA researchers, with no other involvement (e.g., schools releasing student grades), (4) will only have access to or be responsible for analyzing de-identified data. When in doubt, please contact Regulatory Services for further guidance.
Which personnel require IRB approval?
During new protocol submissions, the IRB will review the research team’s qualifications to verify and document all requirements for training, COI disclosures, reliance requirements, etc. After the protocol is approved, only certain subsequent personnel changes require IRB approval via a Modification: the PI, Faculty Advisor, SQP with qualifications different than the original approved personnel, and Non-UTA Personnel. Other personnel changes do not require a Modification for IRB approval, but the PI is responsible for vetting training/COI and maintaining documentation (see Table in Section A).
How do I document addition or removal of protocol personnel with this new process?
Consult the Table in Section A. For personnel changes requiring IRB approval, submit a Modification. For all other personnel changes, consult the Table for your list of responsibilities and necessary documentation. To ensure proper compliance with IRB policies and procedures, all requirements must be met prior to the person beginning work on the project. Documentation may be maintained in paper or electronic form, but it must be available to the IRB upon request. To remove personnel, create a note to file or document the person’s official end date. It is recommended to maintain a personnel tracking list - an example template is available: IRB Personnel Tracking Sheet.
Is it still required to submit a protocol Modification to change the PI, Faculty Advisor, or Non-UTA Personnel?
Yes.
What are Specially Qualified Personnel (SQP)?
Specially Qualified Personnel, or SQP, refers to personnel with special professional qualifications, credentials, licensure, or expertise specific to their role and necessary/integral to the conduct of the research. For example, this could include a physician, research nurse, phlebotomist, radiologist, clinical psychologist, licensed clinical social worker, etc. Graduate and undergraduate students typically are not considered SQP. When changing personnel that are SQP, their qualifications must be the same or comparable as the original approved SQP. If they are not, IRB approval is required via a Modification.
What else do I need to think about when changing personnel?
If the personnel change impacts any approved protocol-related materials, such as the informed consent document or a recruitment flier, a Modification for IRB approval is required. To avoid having to submit subsequent modification requests every time there is a personnel change, it is recommended to utilize only the PI’s or Faculty Advisor’s name and contact information in any protocol materials (such as the informed consent document).
How can protocol personnel obtain access to the protocol in Mentis?
The Mentis team is working behind the scenes to add a new “view access” and “edit access” feature to protocols. This will allow PIs to grant access for a protocol to UTA personnel, or any other individual at UTA. This new feature is expected to roll out in the summer. In the interim, and for non-UTA individuals, please provide personnel with copies of the protocol or its relevant parts.
Section F. Resources
Modifying a Protocol
Multi-Site Research and IRB Reliance
IRB Personnel Tracking Sheet
PI or Faculty Advisor Change Form
Principal Investigator Responsibilities
Faculty Advisor Responsibilities
Human Subject Protection (HSP) Training
List of PHS Compliant Agencies for COI Training
COI Training
Responsible Conduct of Research Training
HIPAA Training
Good Clinical Practice (GCP) Training
Research Conflict of Interest Disclosure
